Dr. Bhupendra Kumar Verma

Present affiliation:
Additional Professor,
Department of Biotechnology,
All India Institute of Medical Sciences (AIIMS),
Ansari Nagar,
New Delhi-110029
Email ID: bverma@gmail.com, bverma@aiims.edu
Phone(O): +91-11-26593617
Lab Website: Dr. Bhupendra Kumar Verma
Teaching and Research Experience:
- Associate Professor, Department of Biotechnology, All India Institute of Medical Sciences, New Delhi, 07/2021 to 06/2024
-
Assistant Professor, Department of Biotechnology, All India Institute of Medical Sciences, New Delhi, 11/2017 to 06/2021
-
Assistant Professor of Microbiology, School of Life Sciences, Sikkim University, Gangtok, Sikkim, 09/2017 to 11/2017
-
Post-doctoral Researcher, Institute of Biotechnology, University of Helsinki, Finland, 01/2011 to 09/2017
- Post-doctoral Researcher, New Jersey Medical School, UMDNJ, New Jersey, United States, 07/2010 to 11/2010
Education:
- PhD. Indian Institute of Science, Bangalore, India, 2010- MSc (Microbiology) Maharshi Dayanand Saraswati University, Ajmer, India, 2002
Research:
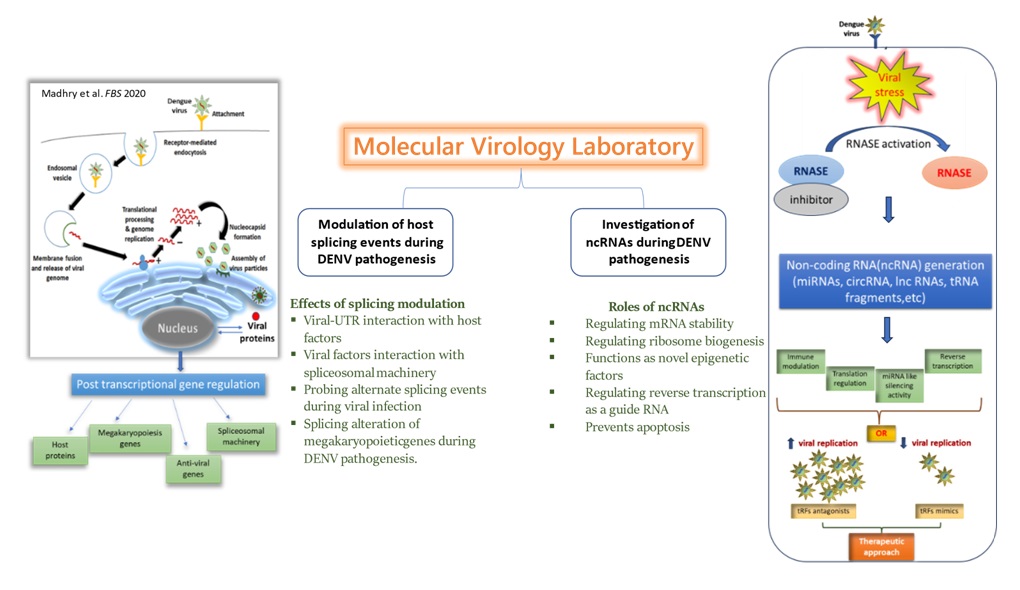
15 years of research experience in the field of Molecular Biology, Virology, Post-transcriptional Gene regulation, RNA splicing etc.
Research Focus: Dengue Virus-host interaction, non-coding RNAs, Alternate Splicing
Characterization of the non-coding RNA interactome in DENV pathophysiology: The global burden of dengue infection poses significant challenges in terms of medical treatment options, as there is a lack of effective therapeutic regimens. This highlights the need to expand our knowledge and deepen our mechanistic understanding of dengue infection in order to develop better strategies for its management. Upon viral infection, multiple signaling cascades are activated, leading to the secretion of various molecules to combat the infection. RNases are among the molecules activated upon infection. Several studies have shown modulation of RNases upon DENV infection. RNases are primarily involved in maintaining RNA homeostasis inside a cell under stressed conditions. They also act upon different RNAs, such as mRNA, tRNA, and rRNA, to generate various non-coding RNAs (ncRNAs). We aim to characterize ncRNA generation during DENV pathogenesis.
-
Modulation of gene regulatory networks in DENV pathophysiology: Although a great deal has been learned about the DENV biology in last decade, there are many regulatory networks of viral-host interactions which are yet to be known. In our lab, we are focusing to map novel regulatory posttranscriptional gene regulation circuits perturbed during pathogenesis of DENV. This will help us to understand how virus manipulates host gene regulation during course of infection and can we target this interaction for therapeutic purpose or as biomarker for severity of disease.
Collaborations
1- Juvenile Nasopharyngeal Angiofibroma: Pathophysiology and Molecular Mechanisms (Department of Otorhinolaryngology, AIIMS, New Delhi):
Juvenile Nasopharyngeal Angiofibroma (JNA) is a benign yet rapidly expanding neoplasm within the posterior nasal cavity and nasopharynx, posing a potentially life-threatening risk due to severe hemorrhage. JNA primarily occurs sporadically and commonly manifests with symptoms such as nasal obstruction, epistaxis (nosebleeds), and related complications due to its location. The molecular and genetic understanding of JNA is still evolving, but research has shed light on certain molecular and genetic factors that may contribute to the development of this rare tumor. We aim to functionally characterize the mutational spectrum and roles of various non-coding RNAs involved in JNA pathogenesis.
2- Characterizing mutations in Indian AT patients (Division of Genetics, Department of Paediatrics, AIIMS, New Delhi):
Ataxia telangiectasia is an autosomal recessive, rare inherited childhood neurological disorder characterized by progressive neurological impairment and cerebellar ataxia. It is caused by mutations in the ATM gene (ataxia telangiectasia mutated). Here, we are characterizing Indian AT patients in terms of their effects on pre-mRNA splicing, NMD decay of mRNA, etc., and aiming to target these mutations with ASO therapy.
Publications:
Google Scholar ID:
https://scholar.google.com/citations?user=VkkZ9xAAAAAJ&hl=en
Scopus ID:
https://orcid.org/0000-0003-1731-5335
- Ravi Kumar, Y.S., Bhavana, A.R., Aziz, A., Roy R., Reddy, A.M., Mahadevappa, P., & Verma, B. (2025). A Comprehensive Review of Ribosomal RNA-Derived Fragments (rRFs): Emerging Insights and Future Directions. International Journal of Biological Macromolecules, (Manuscript in press)
- Singh, A., Sinha, S., & Verma, B. (2025). Dynamic interplay between miR-133a and RBMX during Dengue virus infection. Journal of Medical Virology,, 97(8), e70543. DOI: https://doi.org/10.1002/jmv.70543
- Sokhal, P., Singh, K., Roy, R., Sinha, S., Mehra, S., Ravi Kumar, Y.S., Bhattacharyya, S. & Verma, B. (2025). Megakaryopoiesis Dysregulation, Thrombocytopenia, and Dengue Virus Pathogenesis: Molecular Mechanisms and Therapeutic Advances. Reviews in Medical Virology, 35(4), e70050. DOI:https://doi.org/10.1002/rmv.70050
- Singh, A., Roy, R., Singh, K., Sokhal, P., Afroj, S., Phadnis, S., Ravi Kumar, Y.S., & Verma, B. (2025). Dengue Virus Life Cycle and Host Protein Interactions: Focus on RNA Binding Proteins and Therapeutic Advances. Reviews in Medical Virology, 35(2), e70025. DOI: https://doi.org/10.1002/rmv.70025
- Madhry D, Roy R, Verma B.(2024). Biotin-Based Northern Blotting (BiNoB): A Cost-Efficient Alternative for Detection of Small RNAs. Current Protocols. Dec;4(12):e70065. DOI: https://doi.org/10.1002/cpz1.70065
-
Madhry, D., Kumari, K., Meena, V., Roy, R., & Verma, B. (2024). Unravelling tRNA Fragments in DENV Pathogenesis:
Insights from RNA Sequencing. Scientific Reports, 14(1), 18357.
DOI: https://doi.org/10.1038/s41598-024-69391-7 -
Sinha, S., Singh, K., Ravi Kumar, Y.S., Roy, R., Phadnis, S., Meena, V., Bhattacharyya, S. and Verma, B. (2024).
Dengue virus pathogenesis and host molecular machineries. Journal of Biomedical Science, 31(1), p.43.
DOI: https://doi.org/10.1186/s12929-024-01030-9 -
Kumari, K., Afroj, S., Madhry, D., Verma, Y., Kairo, A, K., Thakar, A., Sikka, K., Verma, H., & Verma, B. (2024).
Comprehensive Analysis of Juvenile Nasopharyngeal Angiofibromas via Whole Exome Sequencing. Genes, Chromosome and Cancer,
DOI: https://doi.org/10.1002/gcc.23265 - Basu, V., Shabnam., Murghai, Y., Ali, M., Sahu, S., Verma, B. K., & Seervi, M. (2024). ONC212, alone or in synergistic conjunction with Navitoclax (ABT-263), promotes cancer cell apoptosis via unconventional mitochondrial-independent caspase-3 activation. Cell Communication and Signaling , 22(1), 1-20. DOI: https://doi.org/10.1186/s12964-024-01817-1
-
Sankar, A., Ravi Kumar Y,S., Singh, A., Roy, R., Shukla, R., & Verma, B. (2024). Next-Generation Therapeutics for Rare
Genetic Disorders. Mutagenesis, 39(1), 157-171.
DOI: https://doi.org/10.1093/mutage/geae002 - Madhry, D., Malvankar, S., Phadnis, S., Srivastava, R. K., Bhattacharyya, S., & Verma, B. (2023).
Synergistic correlation between host angiogenin and Dengue virus replication. RNA Biology, 20(1), 805-816.
DOI: https://doi.org/10.1080/15476286.2023.2264003 - Malvankar, S., Singh, A., Ravi Kumar Y,S., Sahu, S., Shah, M., Murghai, Y., Seervi, M., Srivastava, R, K., & Verma, B. (2023).
Modulation of various host cellular machinery during COVID-19 infection Reviews in Medical Virology, e2481.
DOI: https://doi.org/10.1002/rmv.2481 - Singh, A., Pandey, K.K., Kumar, S., Srivastava, R. K., & Verma, B. (2023).
The SARS CoV-2 UTR’s Intrudes host RBPs and Modulates Cellular Splicing Advances in Virology. Apr 5;2023.
DOI: https://doi.org/10.1155/2023/2995443 - Rai S, Bharti PS, Singh R, Rastogi S, Rani K, Sharma V, Gorai PK, Rani N, Verma BK ,
Reddy TJ, Modi GP, Inampudi KK, Pandey HC, Yadac S, Rajan R, Nikolajeff F, and Kumar S.(2023) Circulating plasma miR-23b-3p as
a biomarker target for idiopathic Parkinson's disease: comparison with small extracellular vesicle miRNA. Frontiers in Neuroscience. 2023;17.
DOI: https://doi.org/10.3389/fnins.2023.1174951 - Singh, A., Malvankar, S., Kumar, Y. R., Seervi, M., Srivastava, R. K., & Verma, B. (2022).
Role of various non-coding RNAs in EMT, cancer, and metastasis: Recent trends and future perspective. Advances in Cancer Biology-Metastasis, 100039.
DOI: https://doi.org/10.1016/j.adcanc.2022.100039 - Siddappa, R. Y., Aditya Rao, S. J., Usha, B. M., Verma, B., & Mahadevappa, P. (2022).
Anti-proliferative Activity of Labdane Diterpenes Isolated from Polyalthia cerasoides and their Molecular Interaction Studies.
Current Drug Discovery Technologies, 19(5), 78-85.
DOI: https://doi.org/10.2174/1570163819666220511154837 - Bhardwaj, A., Sapra, L., Saini, C., Azam, Z., Mishra, P.K., Verma, B.,Mishra GC, Srivastava R.K. (2022).
COVID-19: immunology, immunopathogenesis and potential therapies. International reviews of immunology 2022 Feb 21;41(2):171-206
DOI: https://doi.org/10.1080/08830185.2021.1883600 - Pandey, K.K., Madhry, D., Ravi Kumar Y.S., Malvankar, S., Sapra, L., Srivastava, R., Bhattacharyya, S., and Verma, B
(2021). Regulatory roles of tRNA-derived RNA fragments in human pathophysiology. Molecular Therapy- Nucleic Acids, 26, 161-173.
DOI: https://doi.org/10.1016/j.omtn.2021.06.023 - Madhrey, D., Pandey, K,K., Kaur, J., Rawat, Y., Sapra, Y., Ravikumar Y. S., Srivastava, R.,
Bhattacharyya, S., and Verma, B (2021). Role of non-coding RNAs in Dengue virus-host interaction. Frontiers in Bioscience. 13 (1), 44–55.
DOI: https://doi.org/10.52586/S552 - Sapra, L., Bhardwaj, A., Azam, Z., Madhry, D., Verma, B., Rathore S, Srivastava RK. (2021).
Phytotherapy for treatment of cytokine storm in COVID-19. Frontiers In Bioscience, Landmark.. 2021 Apr 30;26(5):51-75
DOI: https://doi.org/10.52586/4924 - Verma, B., Akinyi, M., Norppa, A., and Frilander, M. J. (2018). Minor splicing and diseases.
Seminar in Cell and Developmental Biology, 79, 103-112.
DOI: https://doi.org/10.1016/j.semcdb.2017.09.036 - Norppa, A., Kauppala, T.M., Heikkinen, H.A., Verma B, Iwai, H. and Frilander, M. J. (2018).
Mutations in the U11/U12-65K protein associated with isolated growth hormone deficiency lead to structural destabilization and impaired binding of
U12 snRNA. RNA, 24(3), 396-409.
DOI: https://doi.org/10.1261/rna.062844.117 - Verbeeren, J., Verma, B., Niemela, EH., Yap, K., Makeyev, EV., and Frilander, MJ. (2017)
Alternate 3’-terminal exon definition events control the choice between retention of U11/U12-65K mRNA in the nucleus and its export to the cytoplasm.
PLOS Genetics. 13, e1006824.
DOI: https://doi.org/10.1371/journal.pgen.1006824 - Argente, J., Flores, R., Gutiérrez-Arumí, A≠., Verma, B≠., Martos-Moreno, G.A., Cuscó, I.,
Oghabian, A., Chowen, J.A., Frilander, M.J., and Pérez-Jurado, L.A. (2014). Defective minor spliceosome mRNA processing results in isolated familial
growth hormone deficiency. EMBO Molecular Medicine. 6 (3), 299-306.
DOI: https://doi.org/10.1002/emmm.201303573
(≠ Equal contribution). - Turunen, J. J., Verma, B., Nyman, T. A., & Frilander, M. J. (2013). HnRNPH1/H2, U1 snRNP, and U11 snRNP cooperate to regulate the
stability of the U11-48K pre-mRNA. RNA, 19(3), 380-389.
DOI: https://doi.org/10.1261/rna.036715.112 - Turunen, J. J., Niemelä, E. H., Verma, B., & Frilander, M. J. (2013). The significant other: splicing by the minor spliceosome.
Wiley Interdisciplinary Reviews: RNA, 4(1), 61-76.
DOI: https://doi.org/10.1002/wrna.1141 - Verma, B., Ponnuswamy, A., Gnanasundram, S.V. and Das, S. (2011). Cryptic AUG is important for 48S ribosomal assembly during internal
initiation of Coxsackievirus B3 RNA. Journal of General Virology. 92, 2310 - 2319.
DOI: https://doi.org/10.1099/vir.0.032151-0 - Verma, B., Bhattacharyya, S. and Das, S. (2010). Polypyrimidine tract binding protein interacts
with Coxsackievirus B3 RNA and influences its translation. Journal of General Virology. 91, 1245-1255.
DOI: https://doi.org/10.1099/vir.0.018507-0 - Bhattacharyya, S1.,Verma, B1., Pandey, G. and Das, S. (2008). The structure and function of a cis-acting element
located upstream of the IRES that influences Coxsackievirus B3 RNA translation. Virology. 377(2):345-354 (Joint first author).
DOI: https://doi.org/10.1016/j.virol.2008.04.019
I am open to discussing and supporting self-motivated, diligent, and talented candidates who are seeking Post-doctoral fellowships or scientist positions funded by agencies such as DBT/DST and ICMR. Prospective candidates are encouraged to reach out via email, providing a 1-page write-up, CV, and three references.
